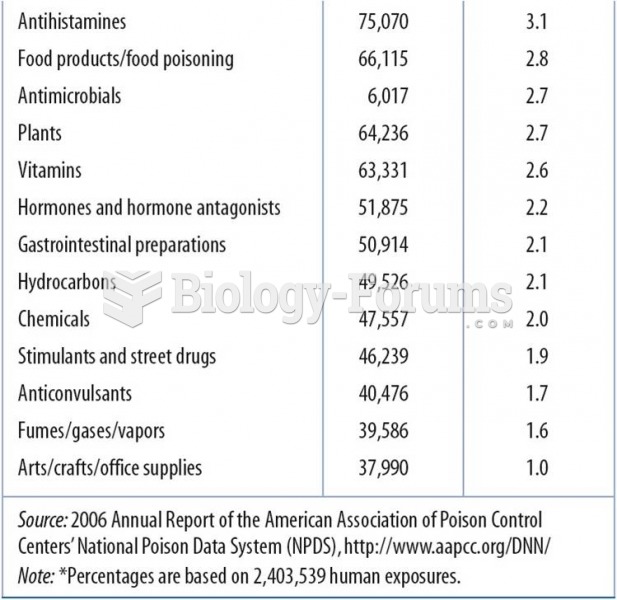
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Did you know?
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Did you know?
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.