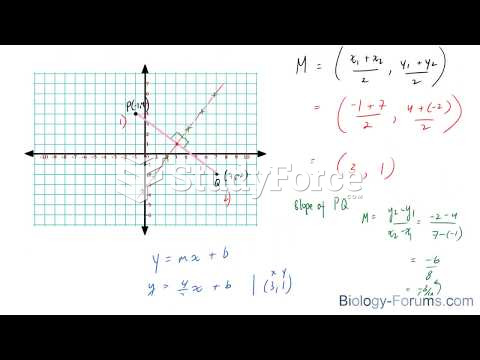
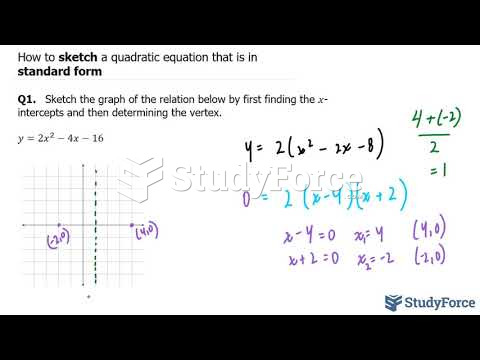
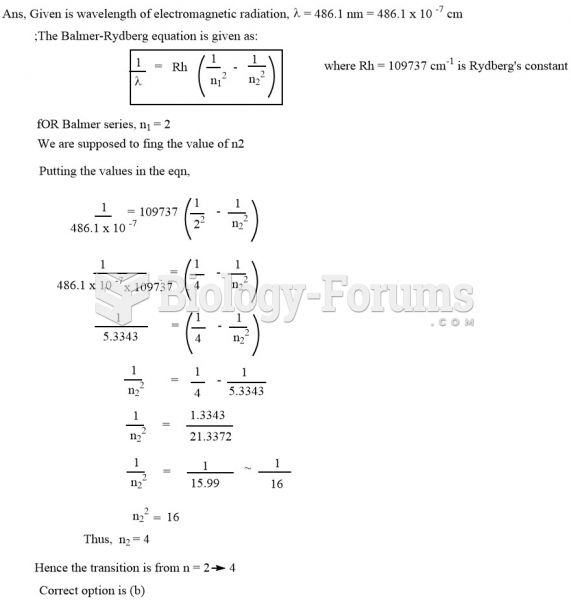
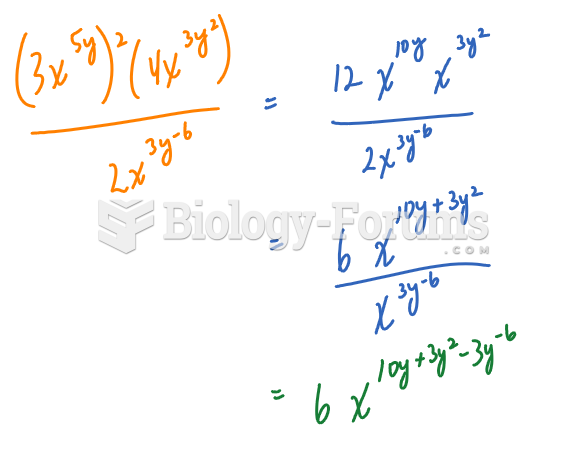|
|
|
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Asthma cases in Americans are about 75% higher today than they were in 1980.
When Gabriel Fahrenheit invented the first mercury thermometer, he called "zero degrees" the lowest temperature he was able to attain with a mixture of ice and salt. For the upper point of his scale, he used 96°, which he measured as normal human body temperature (we know it to be 98.6° today because of more accurate thermometers).
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.