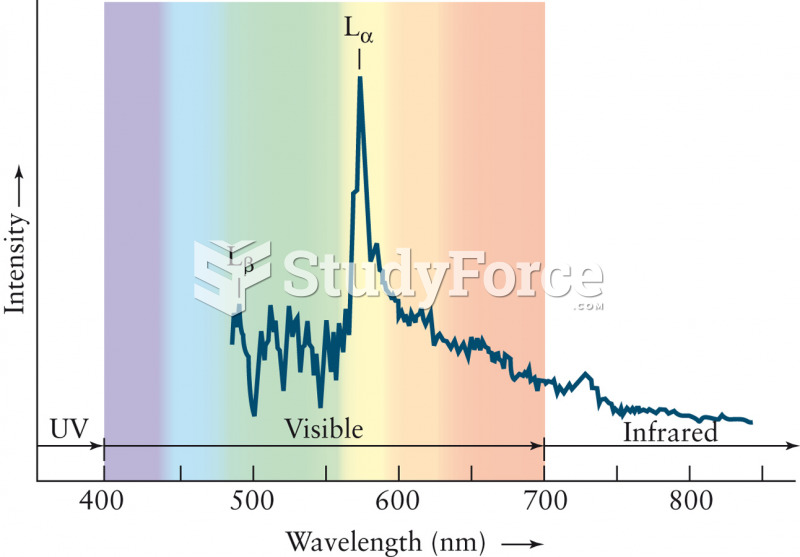
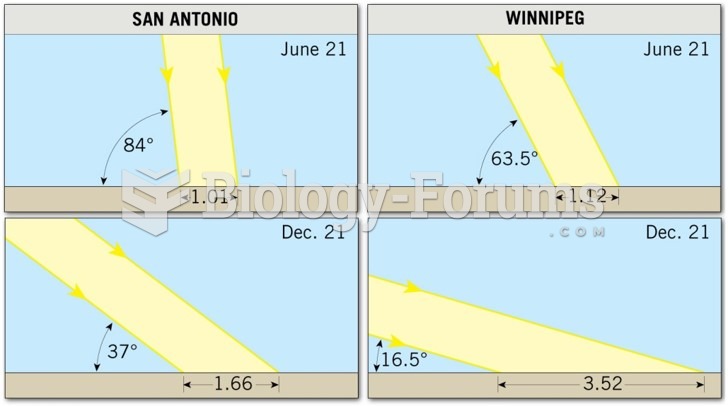
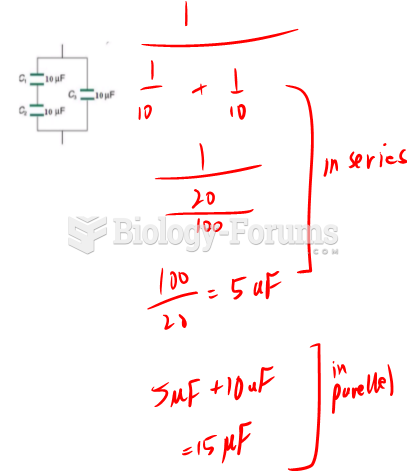
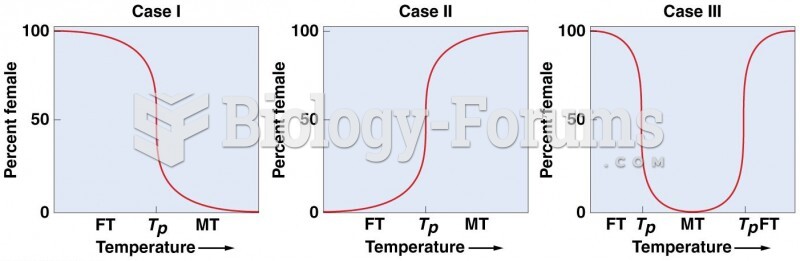
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Did you know?
Methicillin-resistant Staphylococcus aureus or MRSA was discovered in 1961 in the United Kingdom. It if often referred to as a superbug. MRSA infections cause more deaths in the United States every year than AIDS.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.