This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
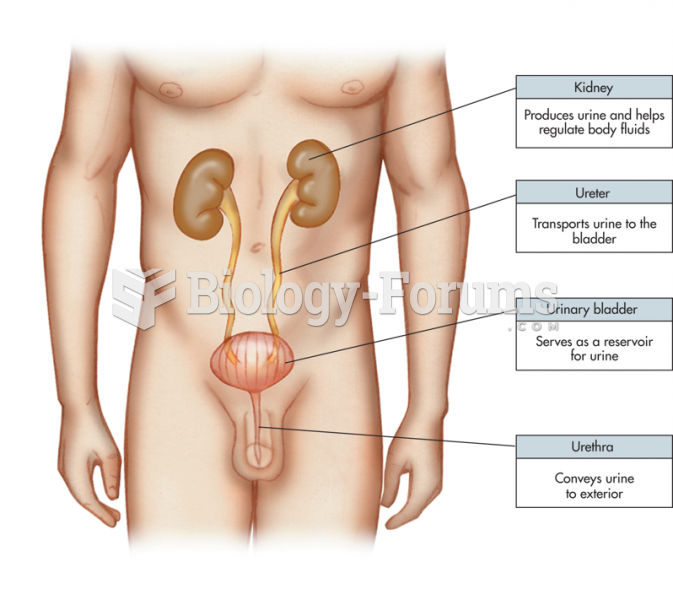
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.
Did you know?
Medication errors are three times higher among children and infants than with adults.
Did you know?
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
Did you know?
As of mid-2016, 18.2 million people were receiving advanced retroviral therapy (ART) worldwide. This represents between 43–50% of the 34–39.8 million people living with HIV.