This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The shortest mature adult human of whom there is independent evidence was Gul Mohammed in India. In 1990, he was measured in New Delhi and stood 22.5 inches tall.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
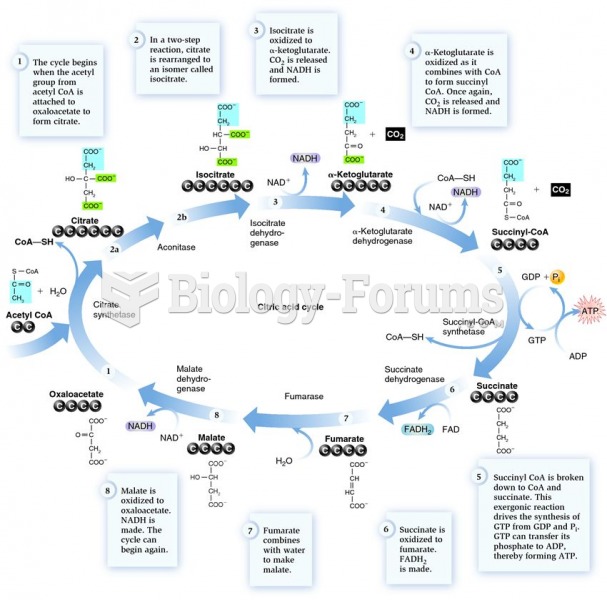
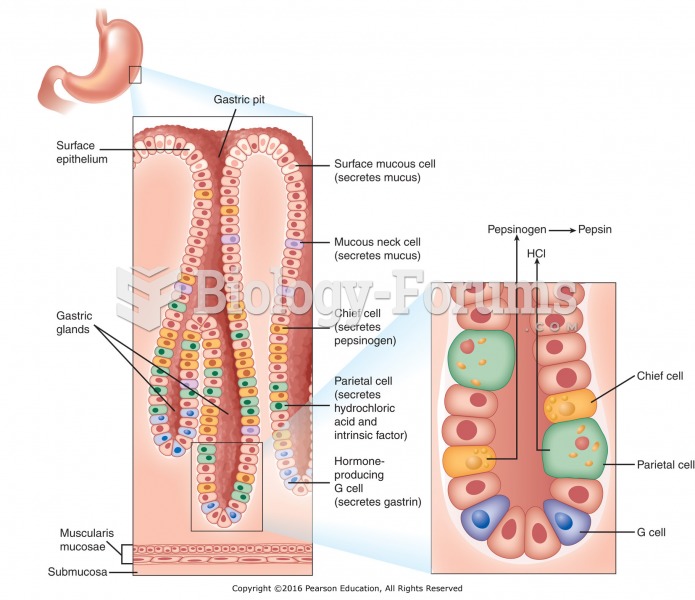
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Blastomycosis is often misdiagnosed, resulting in tragic outcomes. It is caused by a fungus living in moist soil, in wooded areas of the United States and Canada. If inhaled, the fungus can cause mild breathing problems that may worsen and cause serious illness and even death.