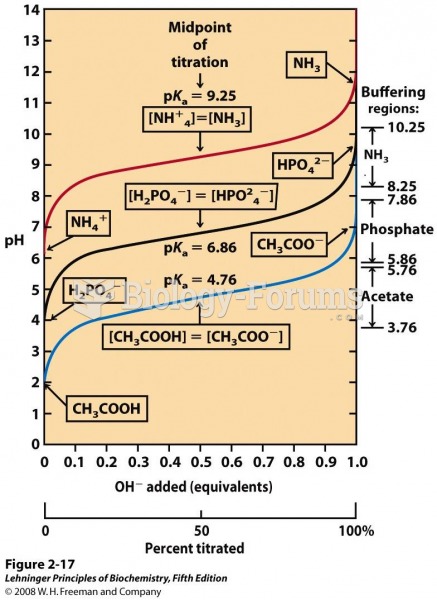
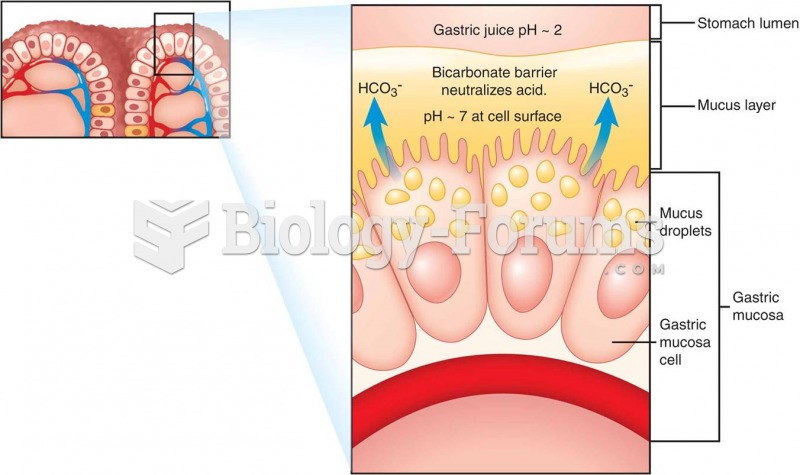
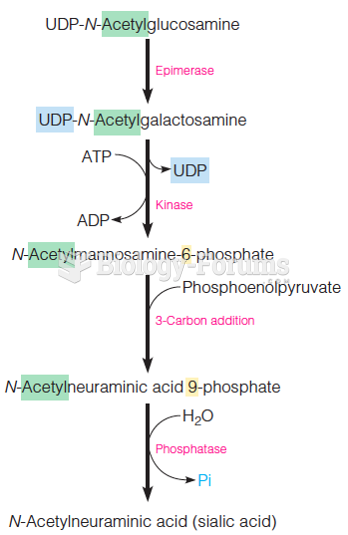
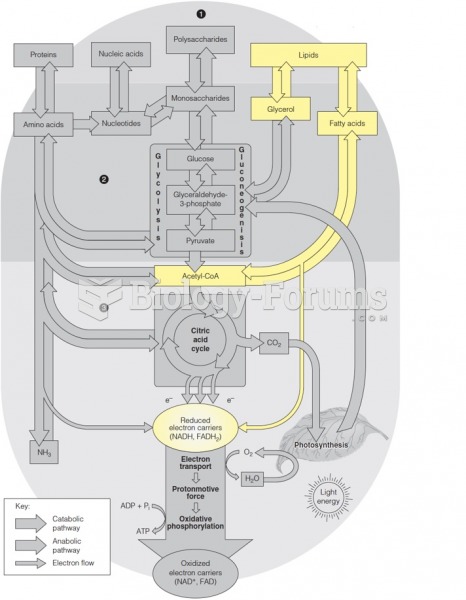
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.