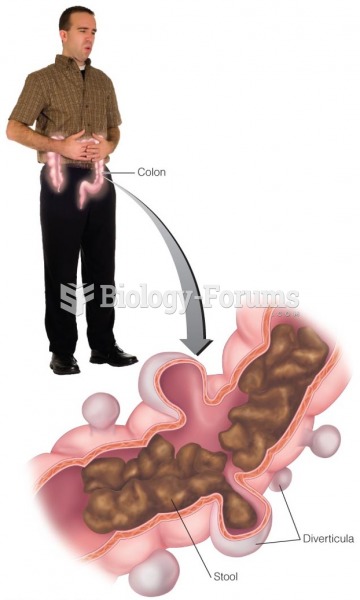
|
|
|
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Oxytocin is recommended only for pregnancies that have a medical reason for inducing labor (such as eclampsia) and is not recommended for elective procedures or for making the birthing process more convenient.
 The nurse needs to inform the patient with impaired vision when a touch is to occur and ask permissi
The nurse needs to inform the patient with impaired vision when a touch is to occur and ask permissi
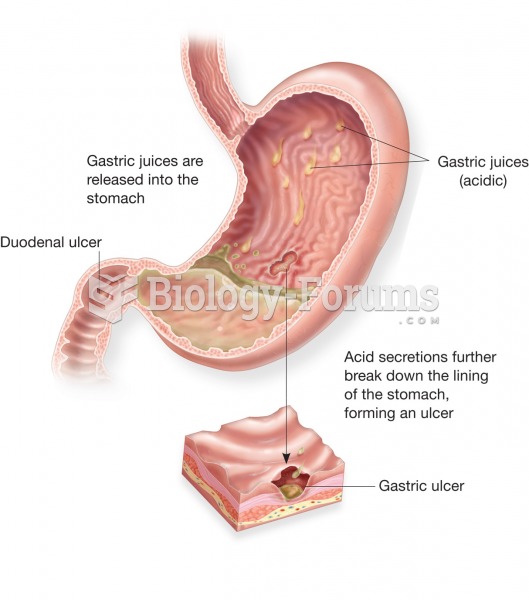 Peptic ulcer. A peptic ulcer may occur in the stomach (gastric ulcer), as shown here, or in the duod
Peptic ulcer. A peptic ulcer may occur in the stomach (gastric ulcer), as shown here, or in the duod