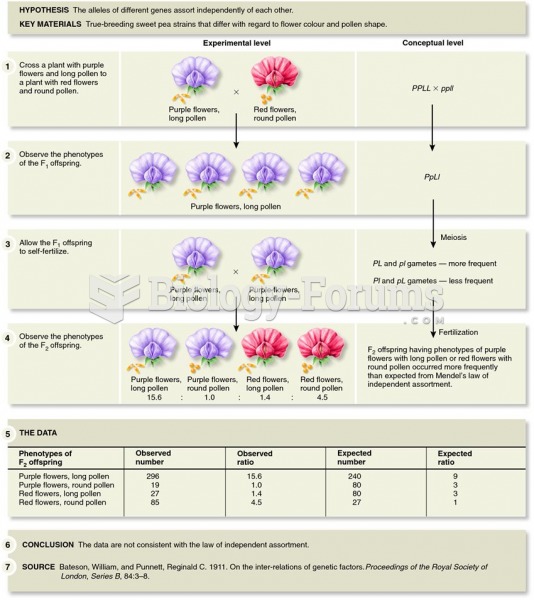
|
|
|
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
A strange skin disease referred to as Morgellons has occurred in the southern United States and in California. Symptoms include slowly healing sores, joint pain, persistent fatigue, and a sensation of things crawling through the skin. Another symptom is strange-looking, threadlike extrusions coming out of the skin.
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
 Mutualisms, such as those that occur among plants and pollinators, generally involve large numbers o
Mutualisms, such as those that occur among plants and pollinators, generally involve large numbers o
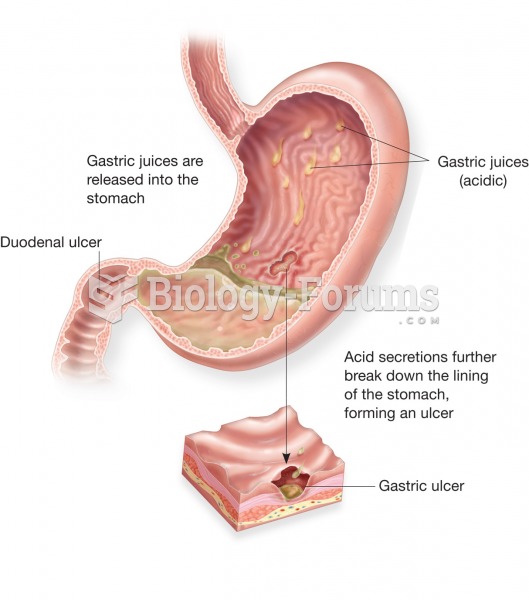 Peptic ulcer. A peptic ulcer may occur in the stomach (gastric ulcer), as shown here, or in the duod
Peptic ulcer. A peptic ulcer may occur in the stomach (gastric ulcer), as shown here, or in the duod
 Scaling the Earth down to the size of a basketball, the Moon is roughly the size of a tennis ball. T
Scaling the Earth down to the size of a basketball, the Moon is roughly the size of a tennis ball. T