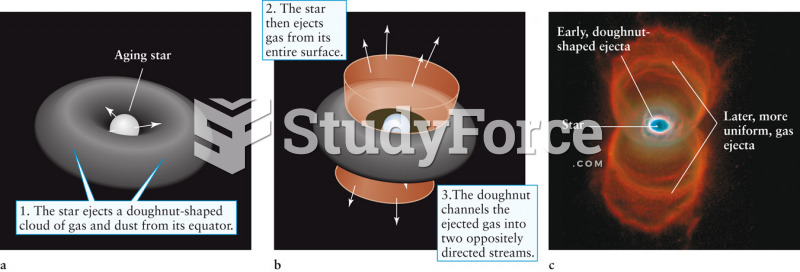
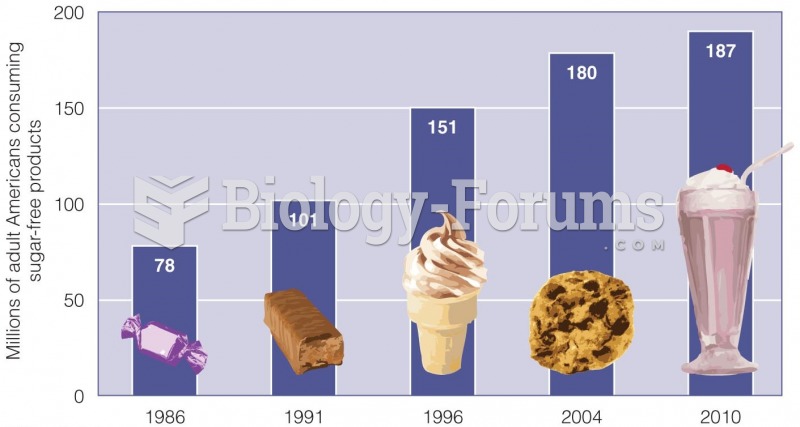
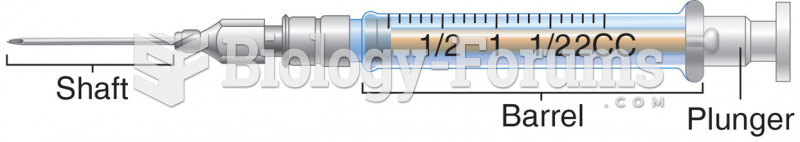
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.