|
|
|
The human body produces and destroys 15 million blood cells every second.
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
There are more nerve cells in one human brain than there are stars in the Milky Way.
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
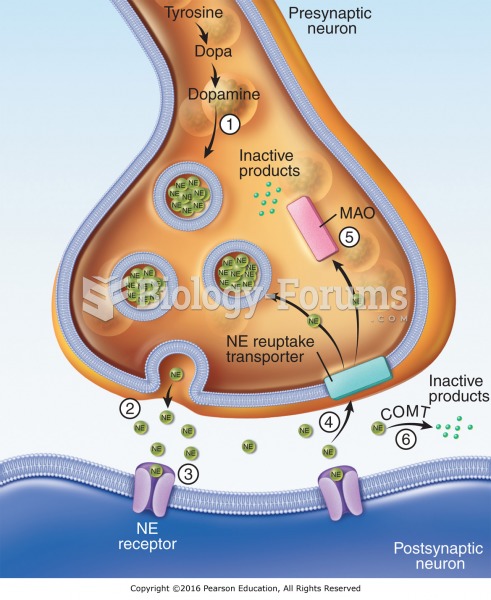 Life cycle of norepinephrine (NE): (1) NE is synthesized from the amino acid tyrosine; (2) NE is ...
Life cycle of norepinephrine (NE): (1) NE is synthesized from the amino acid tyrosine; (2) NE is ...
 (a) A photograph of a radial immunodiffusion reaction for measurement of patient IgA concentrations. ...
(a) A photograph of a radial immunodiffusion reaction for measurement of patient IgA concentrations. ...