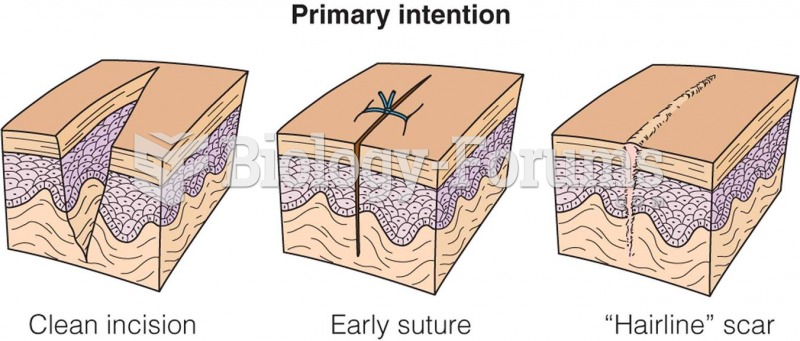
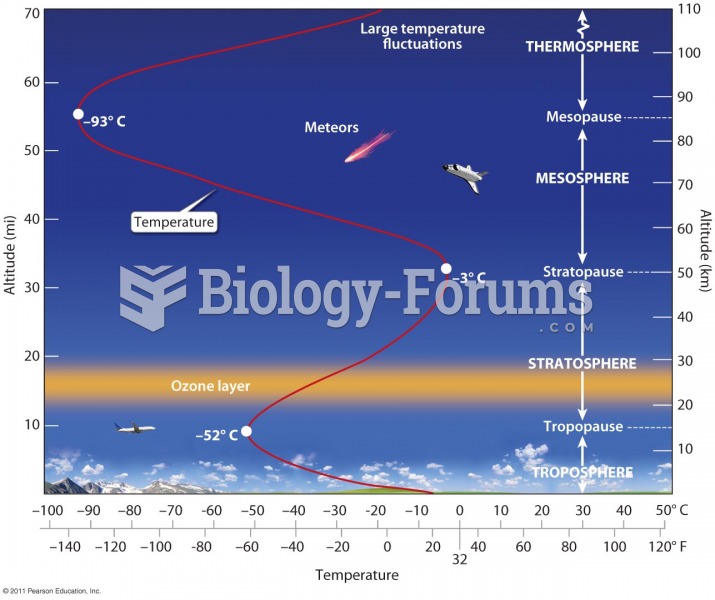
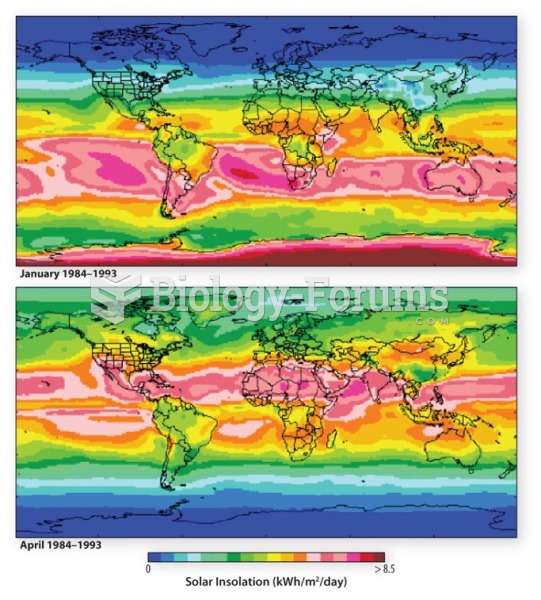
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.