|
|
|
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
 Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
Seasonal changes in biomass and growth form of benthic algae in the Eel River, California: (a) in ea
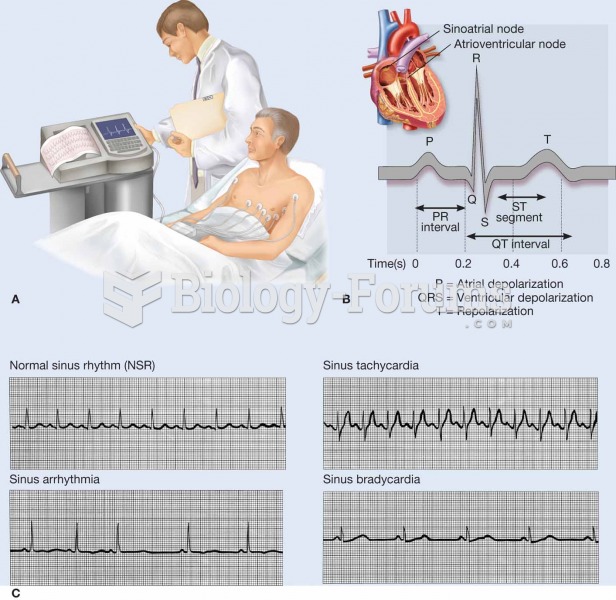 An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat
An electrocardiogram (ECG, EKG) is a commonly used procedure in which the electrical events associat