|
|
|
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.
More than 4.4billion prescriptions were dispensed within the United States in 2016.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
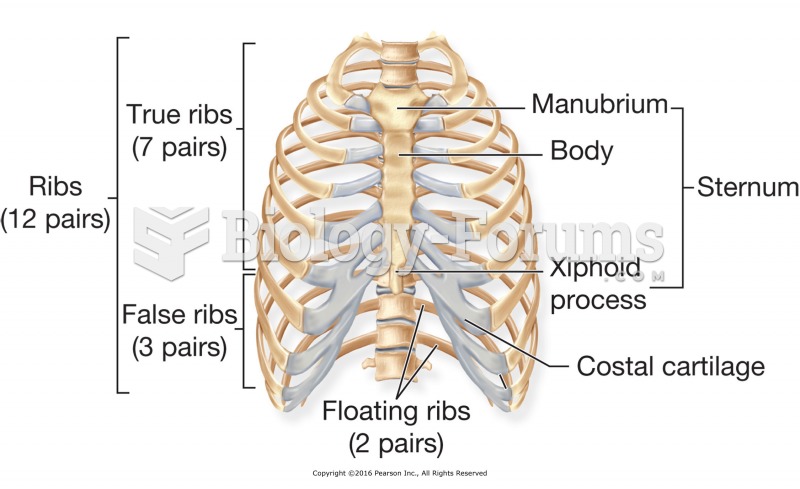
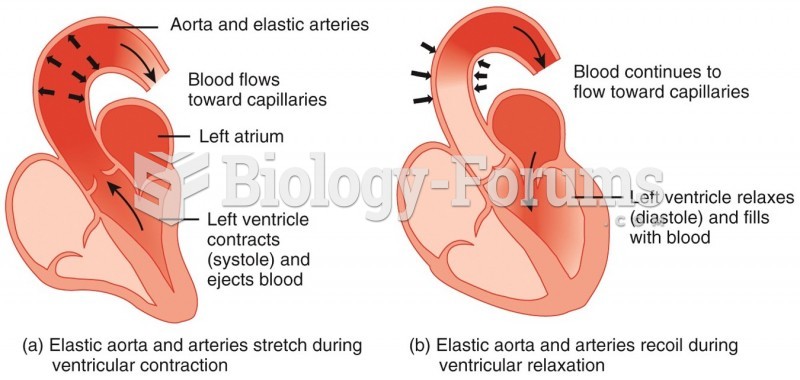
There are 60,000 miles of blood vessels in every adult human.
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.