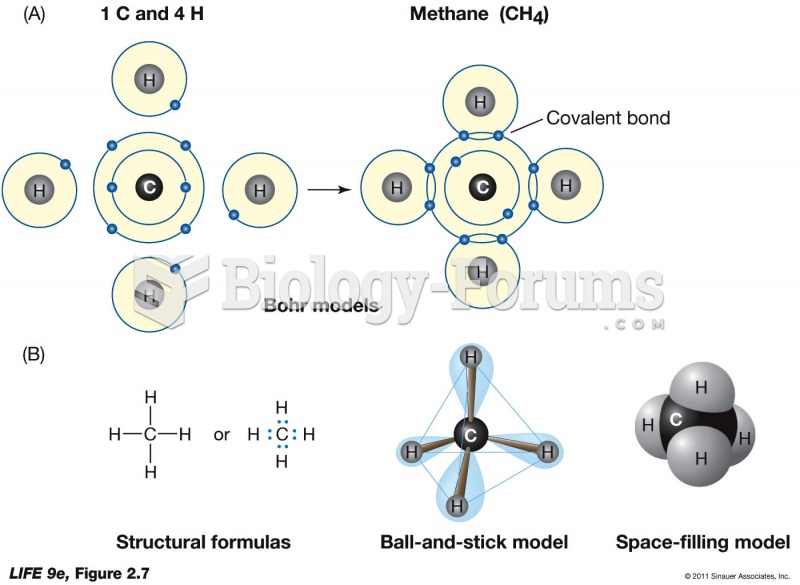
|
|
|
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.
Never take aspirin without food because it is likely to irritate your stomach. Never give aspirin to children under age 12. Overdoses of aspirin have the potential to cause deafness.
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.