|
|
|
The shortest mature adult human of whom there is independent evidence was Gul Mohammed in India. In 1990, he was measured in New Delhi and stood 22.5 inches tall.
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Many supplement containers do not even contain what their labels say. There are many documented reports of products containing much less, or more, that what is listed on their labels. They may also contain undisclosed prescription drugs and even contaminants.
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
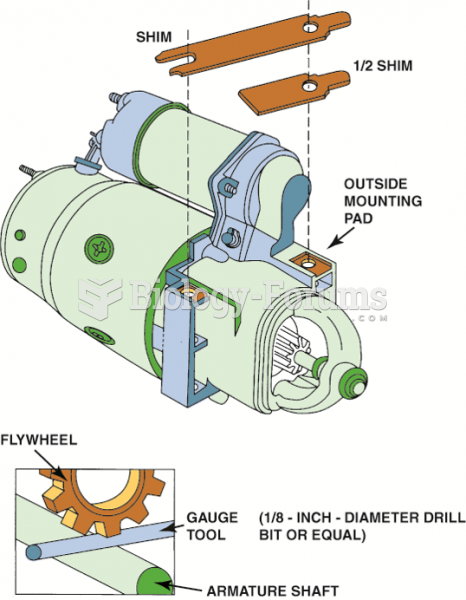 A shim (or half shim) may be needed to provide the proper clearance between the flywheel teeth of ...
A shim (or half shim) may be needed to provide the proper clearance between the flywheel teeth of ...
 This five-wire mass air flow sensor consists of a metal foil sensing unit, an intake air temperature ...
This five-wire mass air flow sensor consists of a metal foil sensing unit, an intake air temperature ...