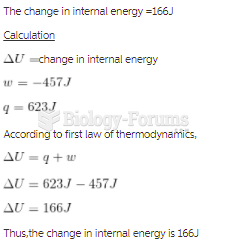This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Did you know?
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
Did you know?
The average adult has about 21 square feet of skin.