|
|
|
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
Though Candida and Aspergillus species are the most common fungal pathogens causing invasive fungal disease in the immunocompromised, infections due to previously uncommon hyaline and dematiaceous filamentous fungi are occurring more often today. Rare fungal infections, once accurately diagnosed, may require surgical debridement, immunotherapy, and newer antifungals used singly or in combination with older antifungals, on a case-by-case basis.
 The world has been horrified recently at a U.S. Congress so polarized and paralyzed that it cannot p
The world has been horrified recently at a U.S. Congress so polarized and paralyzed that it cannot p
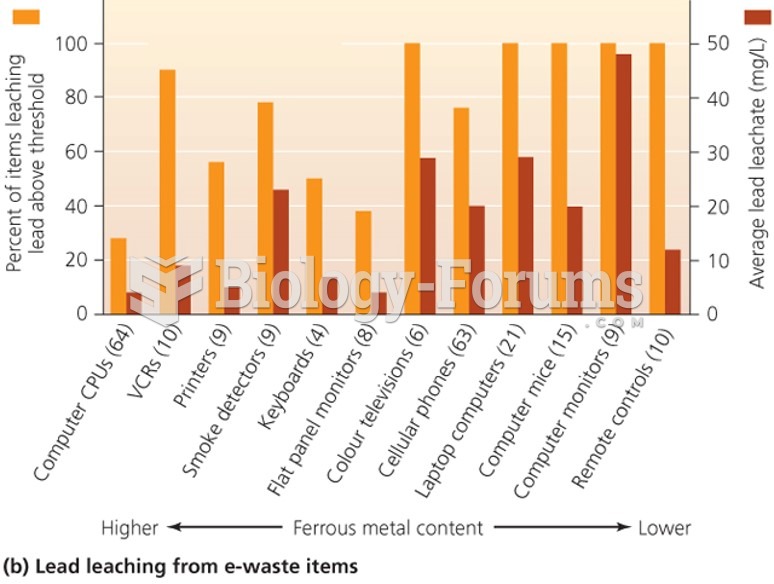
 Some playgrounds near streets with heavy traffic may still have high levels of lead from gasoline ...
Some playgrounds near streets with heavy traffic may still have high levels of lead from gasoline ...