This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The top five reasons that children stay home from school are as follows: colds, stomach flu (gastroenteritis), ear infection (otitis media), pink eye (conjunctivitis), and sore throat.
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Did you know?
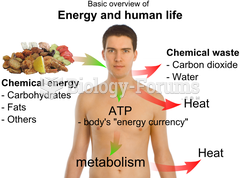
Your heart beats over 36 million times a year.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.
Did you know?
The human body produces and destroys 15 million blood cells every second.