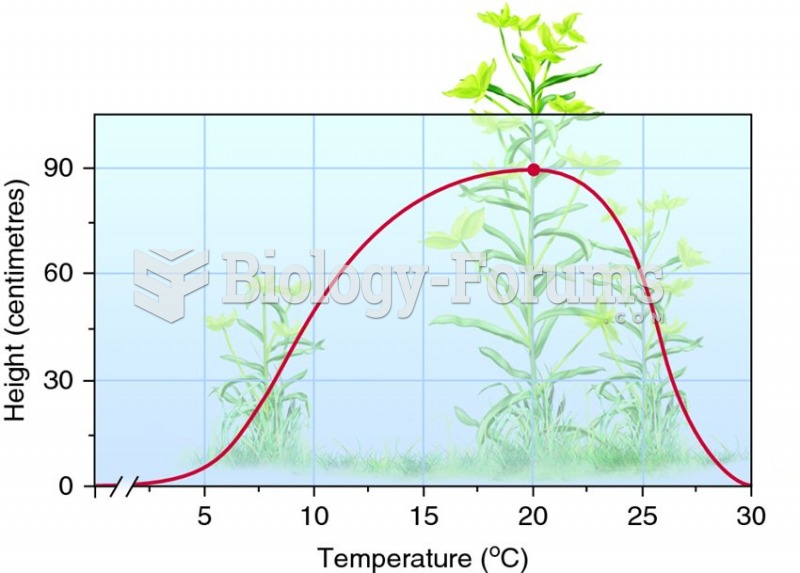
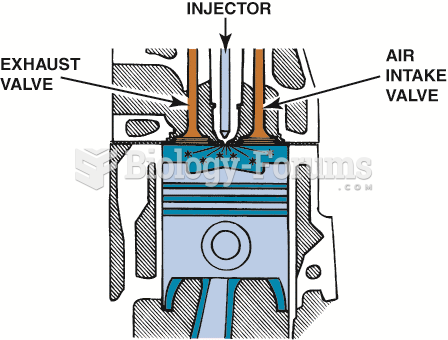
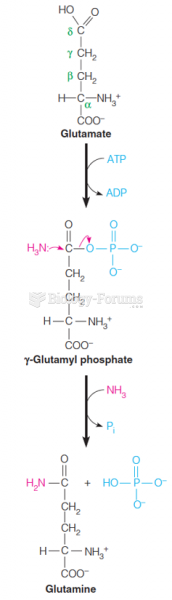
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.
Did you know?
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.