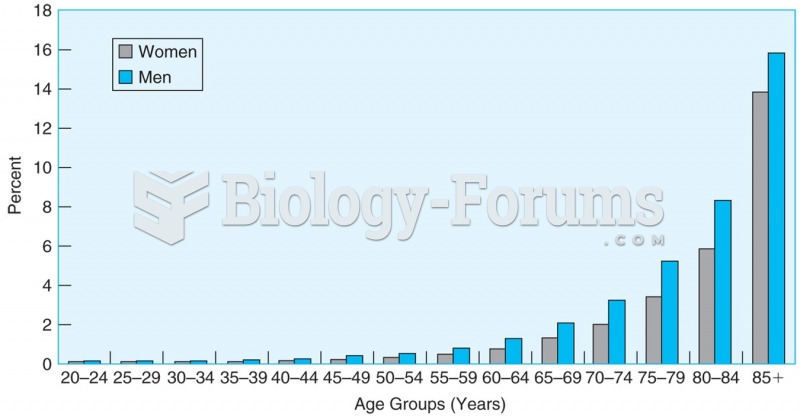
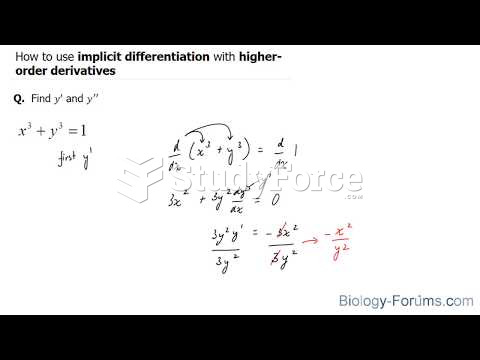
|
|
|
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
Complications of influenza include: bacterial pneumonia, ear and sinus infections, dehydration, and worsening of chronic conditions such as asthma, congestive heart failure, or diabetes.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
 These illustrations show Ellen Craft, a slave with and without a disguise. She dressed “as a disting
These illustrations show Ellen Craft, a slave with and without a disguise. She dressed “as a disting
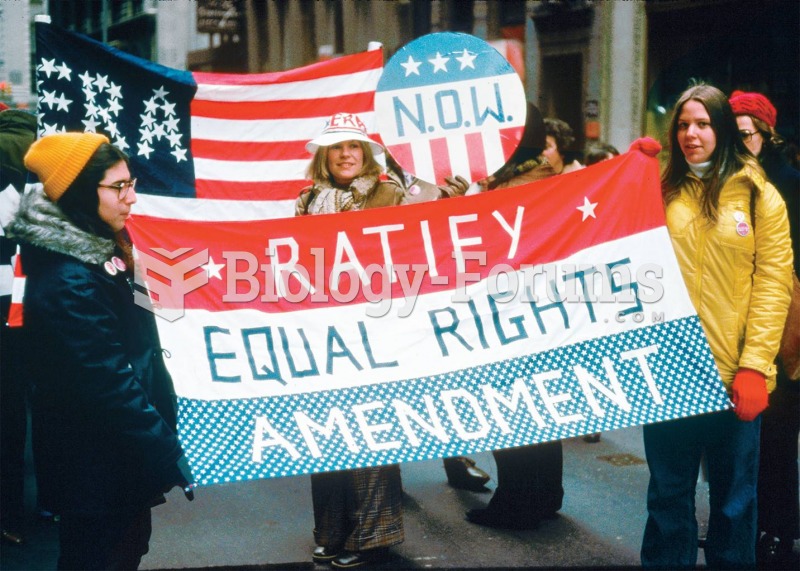 The National Organization for Women holds a rally in Illinois for the Equal Rights Amendment. In ...
The National Organization for Women holds a rally in Illinois for the Equal Rights Amendment. In ...