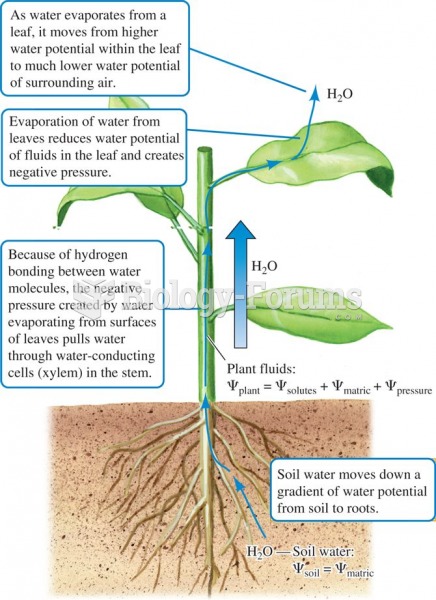
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
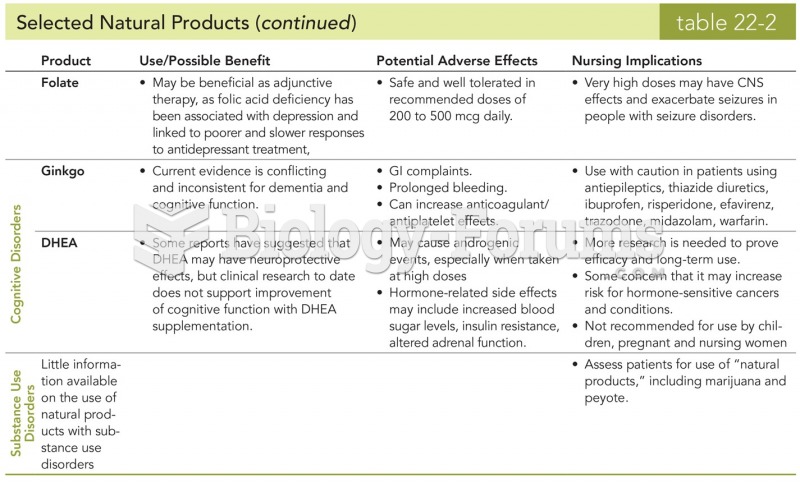
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.