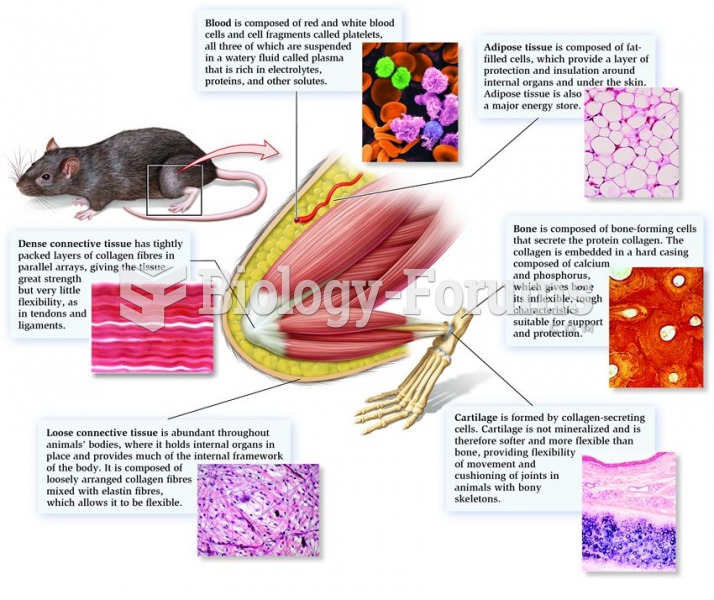
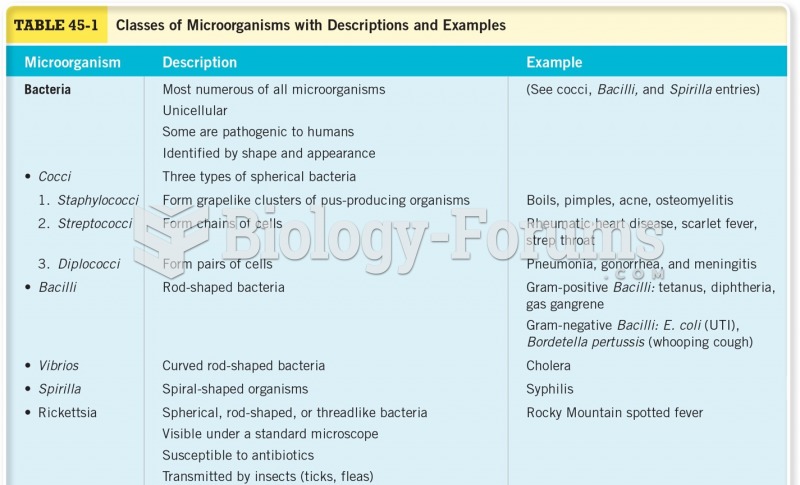
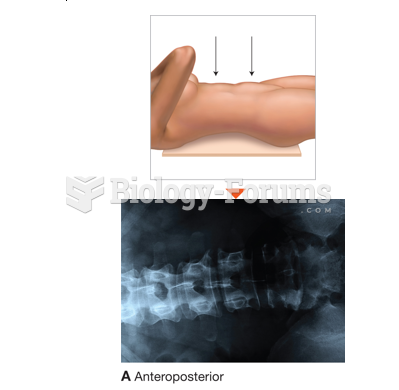
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Did you know?
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.