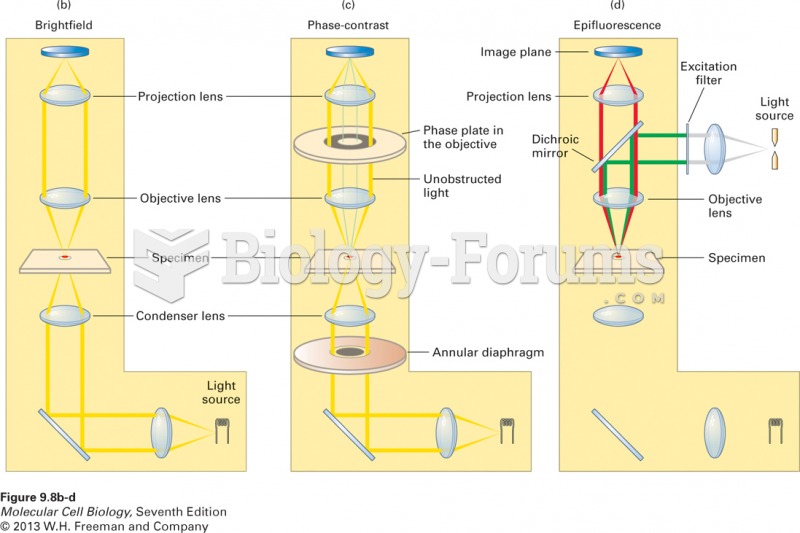
|
|
|
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
 Carnivorous plants, such as this pitcher plant (Sarracenia purpurea) are commonly found in bog habit
Carnivorous plants, such as this pitcher plant (Sarracenia purpurea) are commonly found in bog habit
 This portrait of Thomas Jefferson is considered an accurate likeness, painted when he was thirty-sev
This portrait of Thomas Jefferson is considered an accurate likeness, painted when he was thirty-sev