This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Atropine was named after the Greek goddess Atropos, the oldest and ugliest of the three sisters known as the Fates, who controlled the destiny of men.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Alzheimer's disease affects only about 10% of people older than 65 years of age. Most forms of decreased mental function and dementia are caused by disuse (letting the mind get lazy).
Did you know?
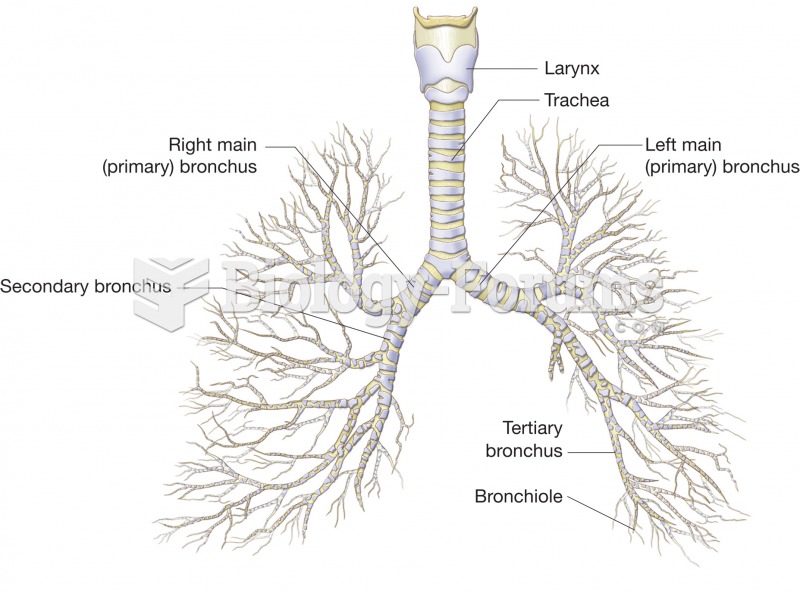
Asthma occurs in one in 11 children and in one in 12 adults. African Americans and Latinos have a higher risk for developing asthma than other groups.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.