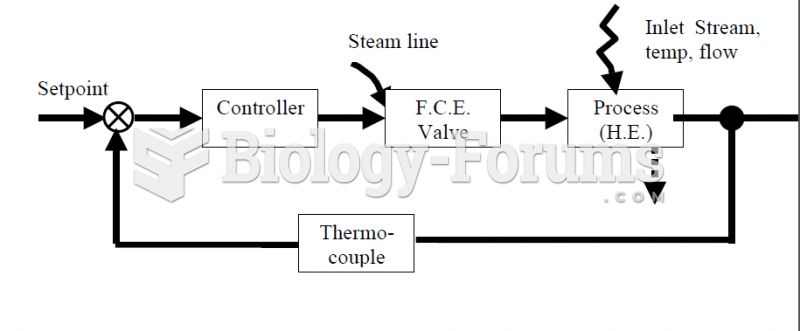
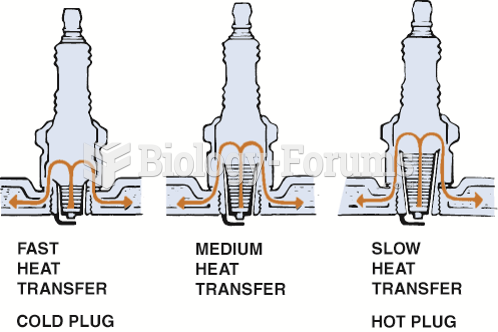
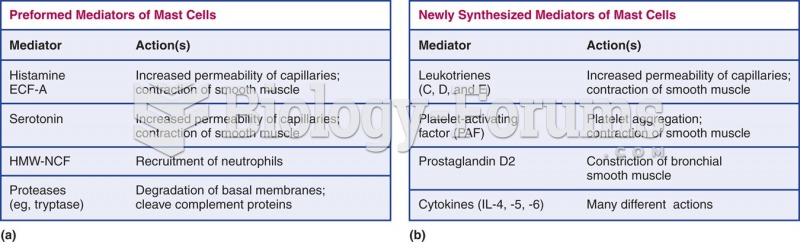
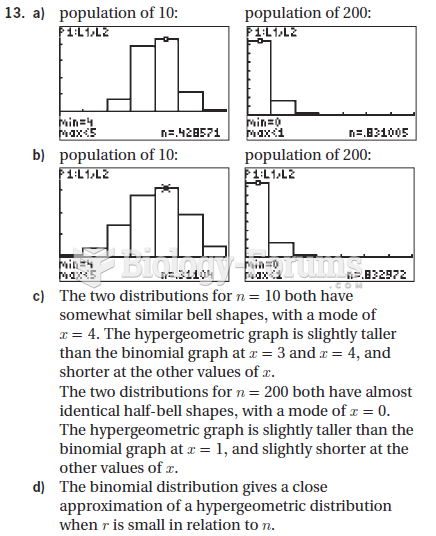
|
|
|
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
This year, an estimated 1.4 million Americans will have a new or recurrent heart attack.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
In 1864, the first barbiturate (barbituric acid) was synthesized.