|
|
|
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
Liver spots have nothing whatsoever to do with the liver. They are a type of freckles commonly seen in older adults who have been out in the sun without sufficient sunscreen.
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
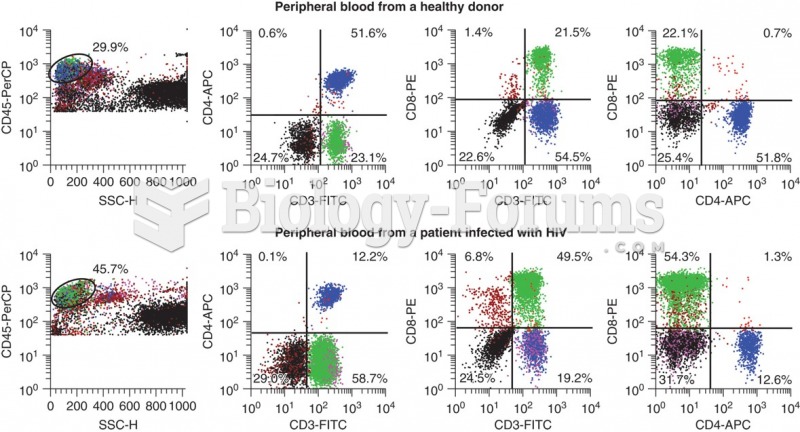 Flow cytometry data that shows the differences in CD3+ CD4+ cells that occur with HIV infection. ...
Flow cytometry data that shows the differences in CD3+ CD4+ cells that occur with HIV infection. ...
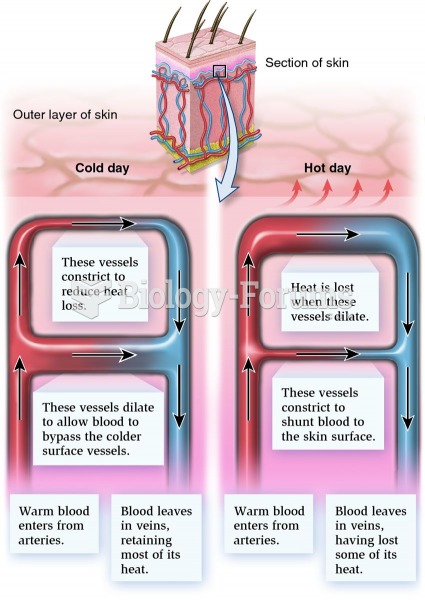
 Get feedback every 5 to 7 minutes about heat intensity. Add or subtract heat barriers to maintain ...
Get feedback every 5 to 7 minutes about heat intensity. Add or subtract heat barriers to maintain ...