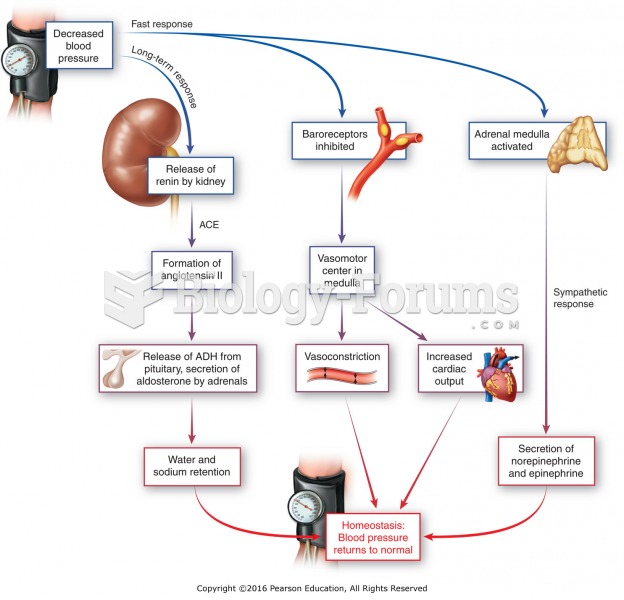
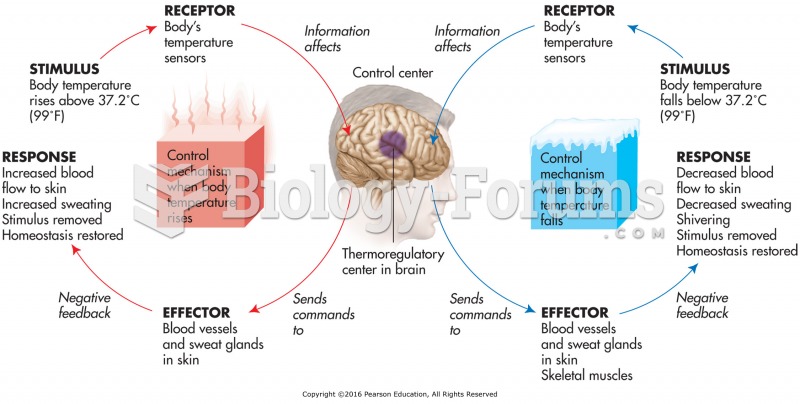
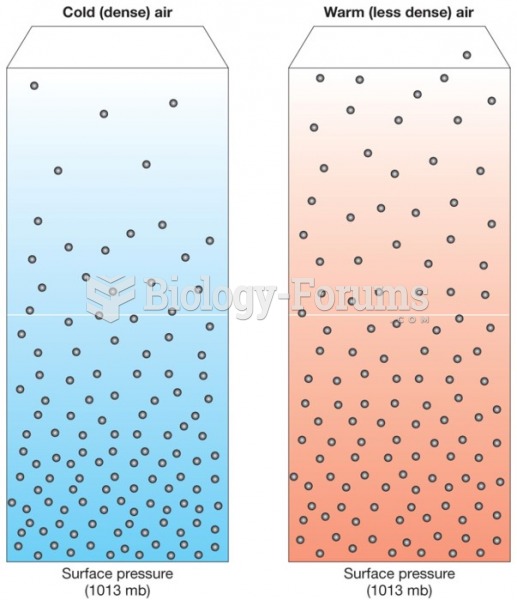
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
Anti-aging claims should not ever be believed. There is no supplement, medication, or any other substance that has been proven to slow or stop the aging process.
Did you know?
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.