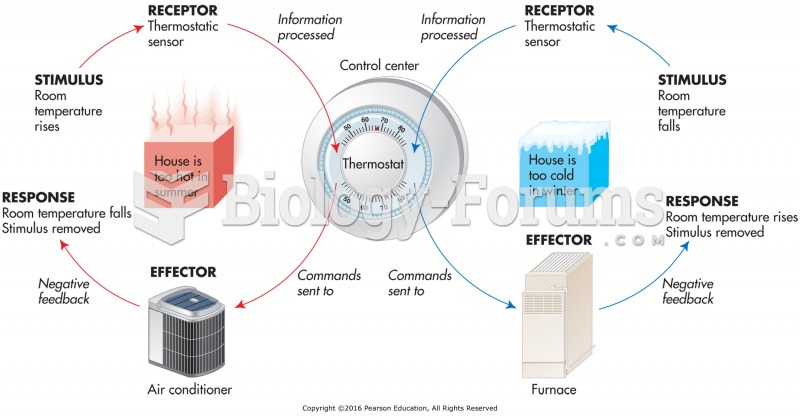
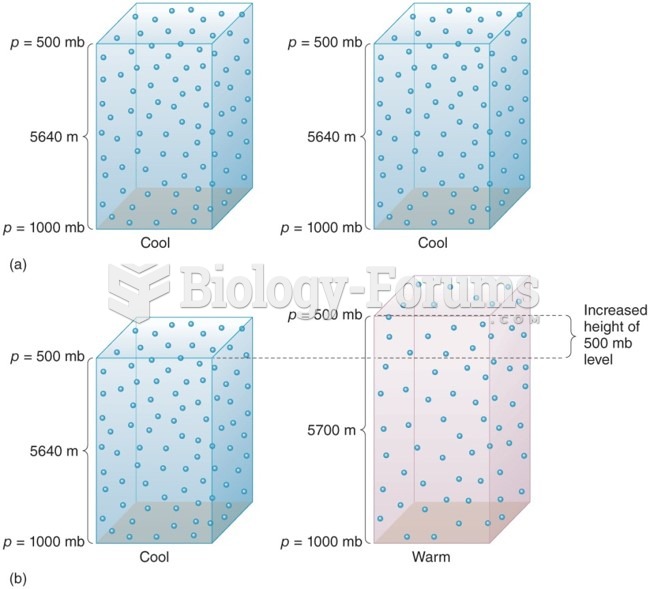
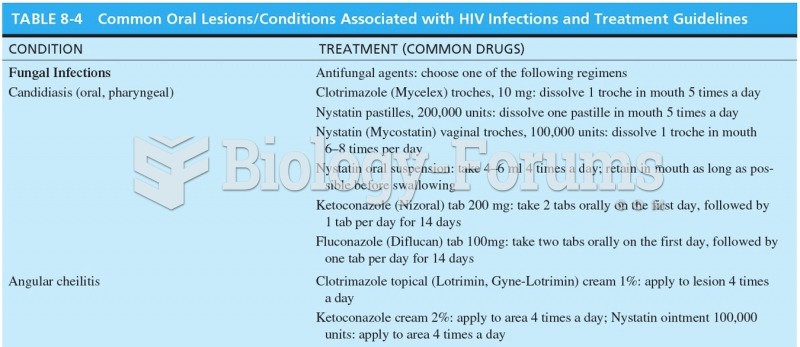
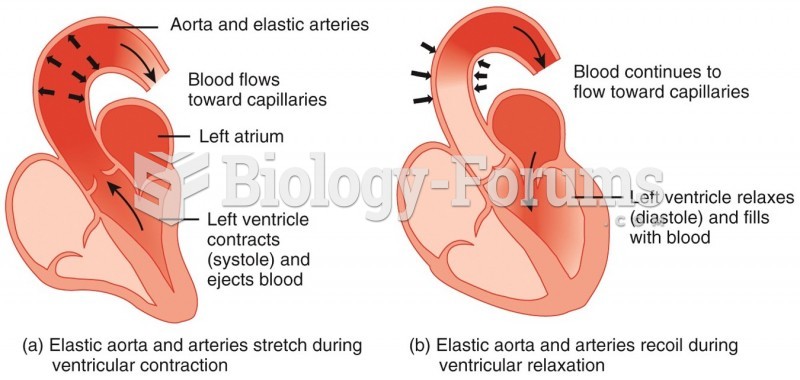
|
|
|
Vaccines prevent between 2.5 and 4 million deaths every year.
Green tea is able to stop the scent of garlic or onion from causing bad breath.
There can actually be a 25-hour time difference between certain locations in the world. The International Date Line passes between the islands of Samoa and American Samoa. It is not a straight line, but "zig-zags" around various island chains. Therefore, Samoa and nearby islands have one date, while American Samoa and nearby islands are one day behind. Daylight saving time is used in some islands, but not in others—further shifting the hours out of sync with natural time.
There are approximately 3 million unintended pregnancies in the United States each year.
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.