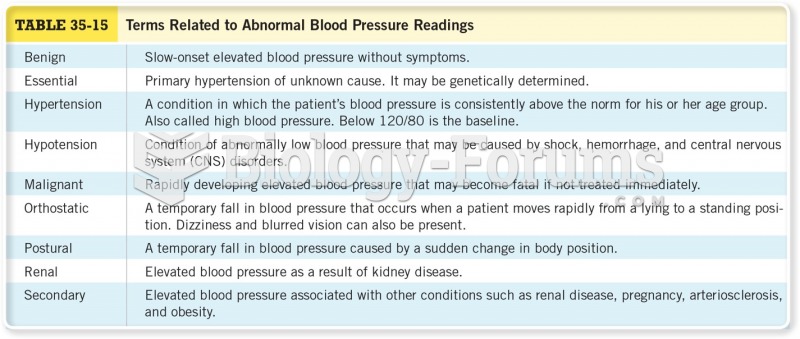
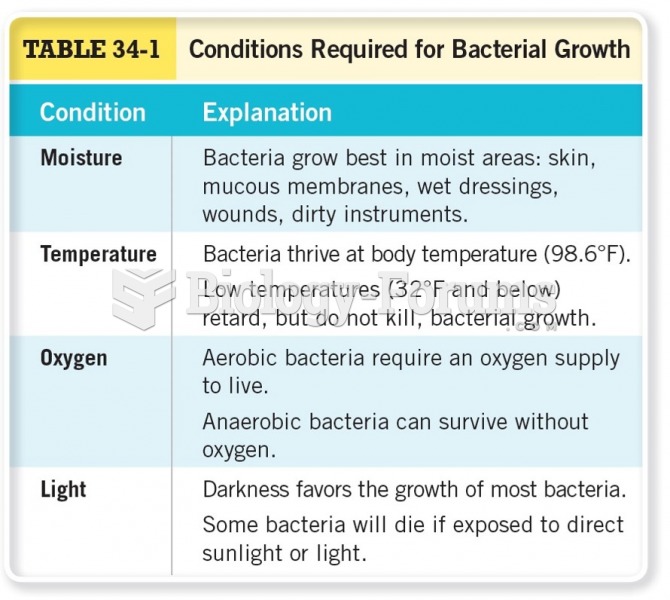
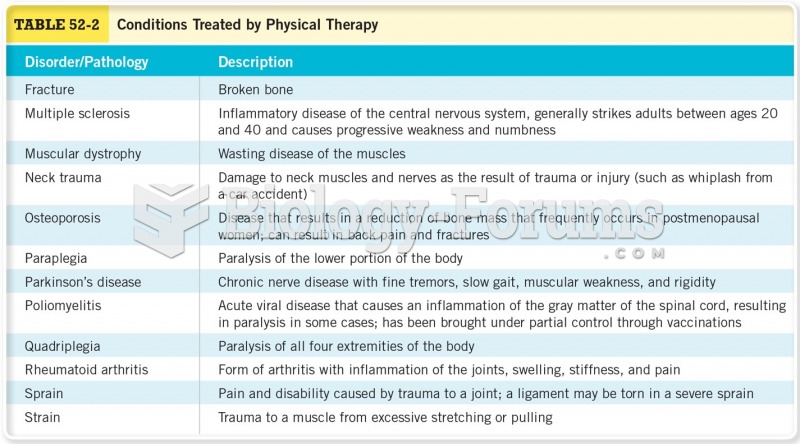
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
According to research, pregnant women tend to eat more if carrying a baby boy. Male fetuses may secrete a chemical that stimulates their mothers to step up her energy intake.
Did you know?
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.