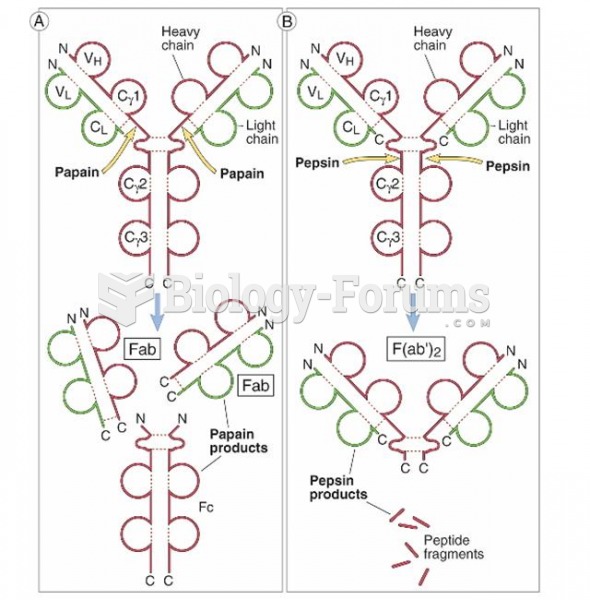
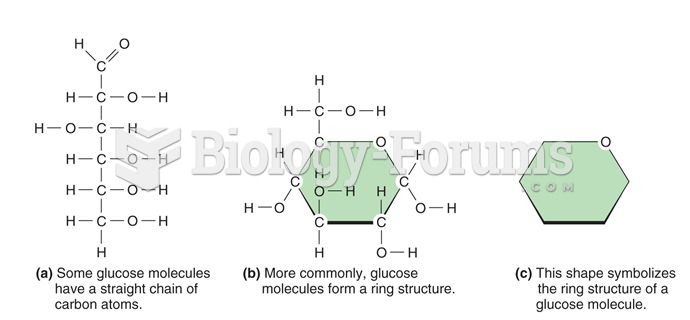
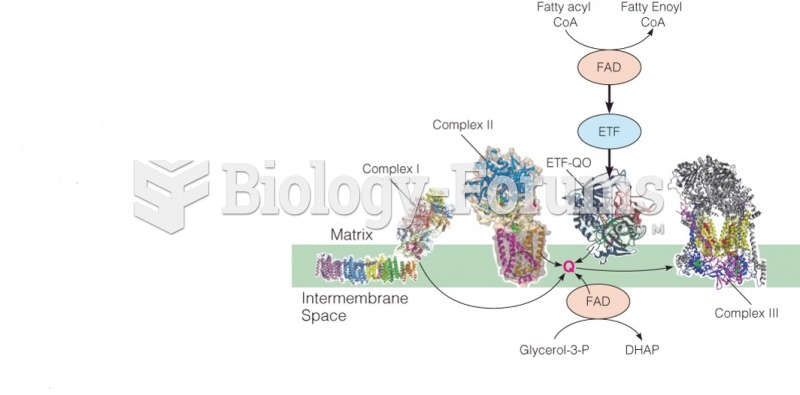
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
The U.S. Pharmacopeia Medication Errors Reporting Program states that approximately 50% of all medication errors involve insulin.
Did you know?
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Did you know?
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.