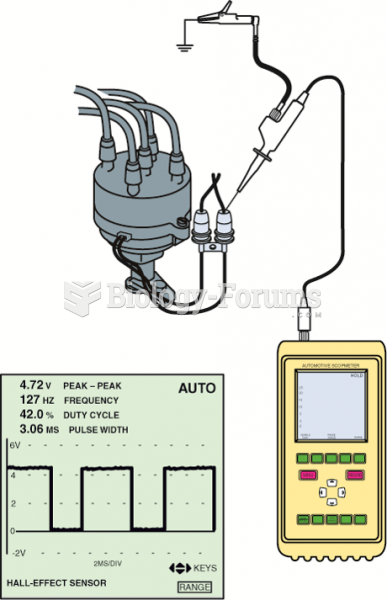
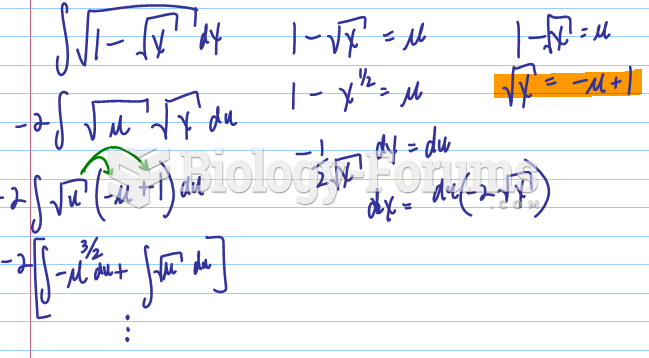
|
|
|
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
 Blocked at every turn by congressional Republicans who hated him, President Obama turned to techniqu
Blocked at every turn by congressional Republicans who hated him, President Obama turned to techniqu
 Each year, the leaders of the world’s eight most powerful nations meet in a secluded place to make ...
Each year, the leaders of the world’s eight most powerful nations meet in a secluded place to make ...