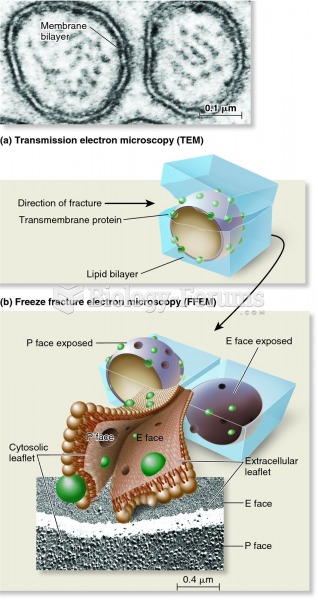
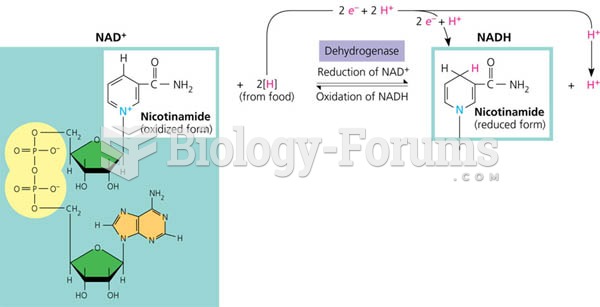
|
|
|
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
When taking monoamine oxidase inhibitors, people should avoid a variety of foods, which include alcoholic beverages, bean curd, broad (fava) bean pods, cheese, fish, ginseng, protein extracts, meat, sauerkraut, shrimp paste, soups, and yeast.