|
|
|
Did you know?
As the western states of America were settled, pioneers often had to drink rancid water from ponds and other sources. This often resulted in chronic diarrhea, causing many cases of dehydration and death that could have been avoided if clean water had been available.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
Did you know?
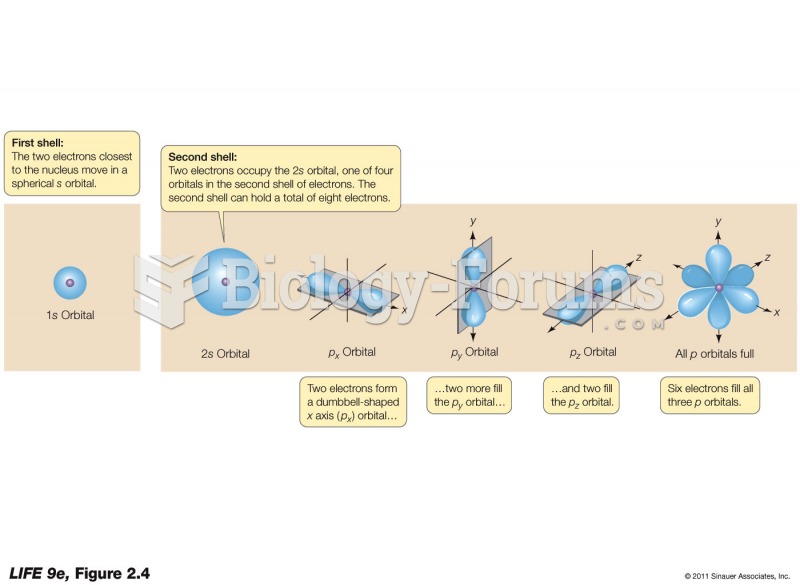
The horizontal fraction bar was introduced by the Arabs.