|
|
|
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Did you know?
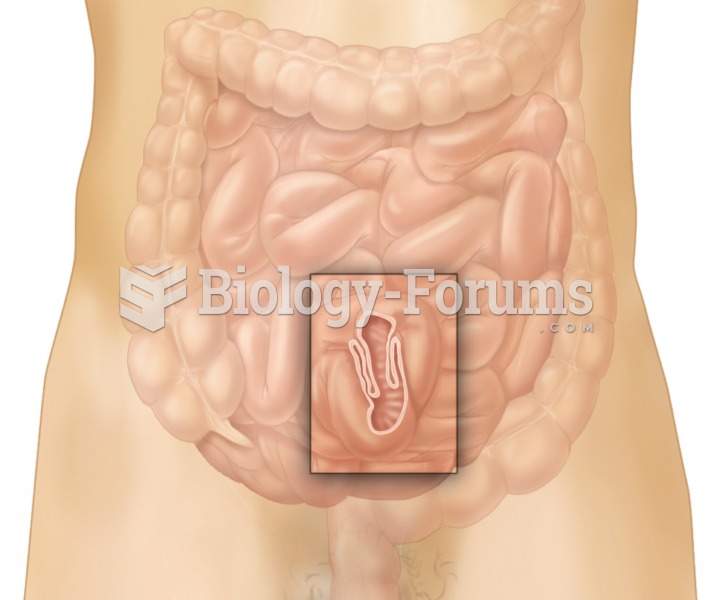
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
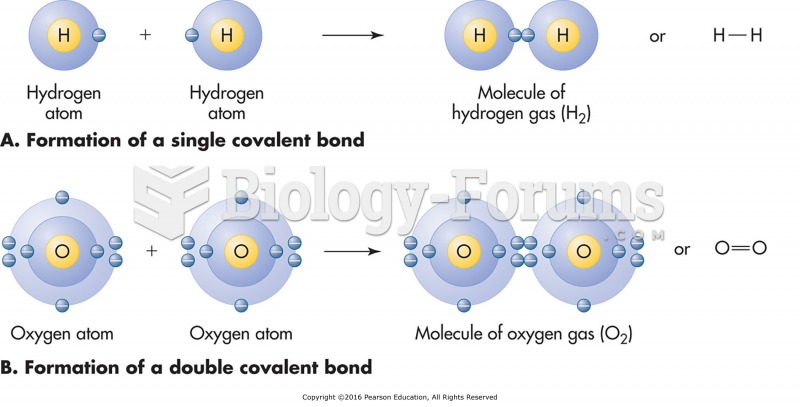
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.