|
|
|
ACTH levels are normally highest in the early morning (between 6 and 8 A.M.) and lowest in the evening (between 6 and 11 P.M.). Therefore, a doctor who suspects abnormal levels looks for low ACTH in the morning and high ACTH in the evening.
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.
 Women have the role of kinkeeper in almost all cultures. This may cause them to react to stress by ...
Women have the role of kinkeeper in almost all cultures. This may cause them to react to stress by ...
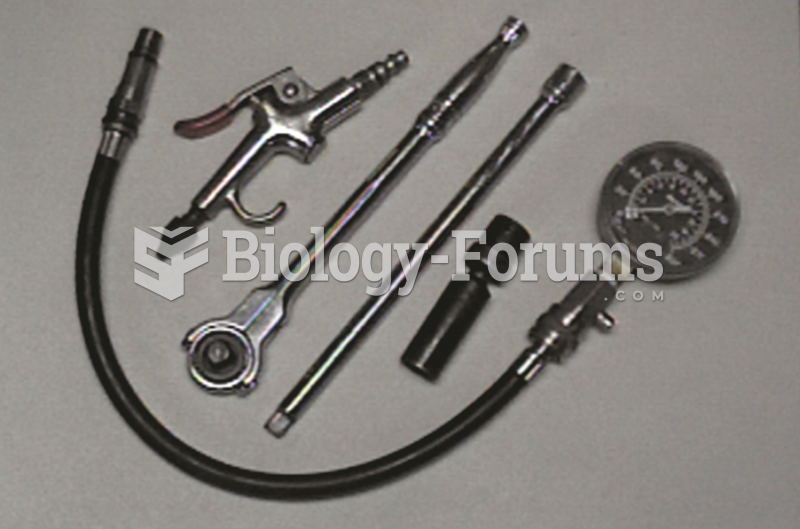 The tools and equipment needed to perform a compression test include a compression gauge, an air ...
The tools and equipment needed to perform a compression test include a compression gauge, an air ...