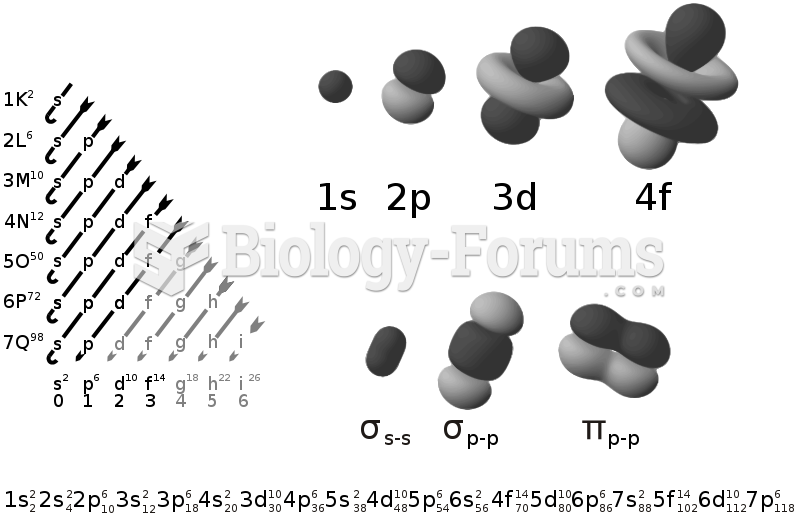
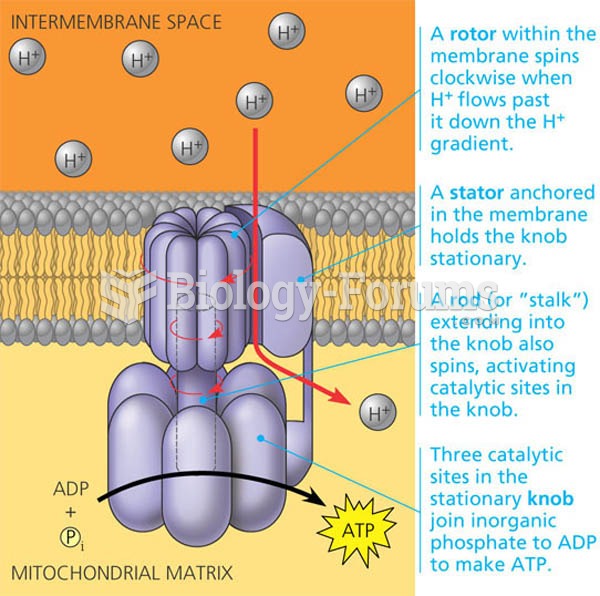
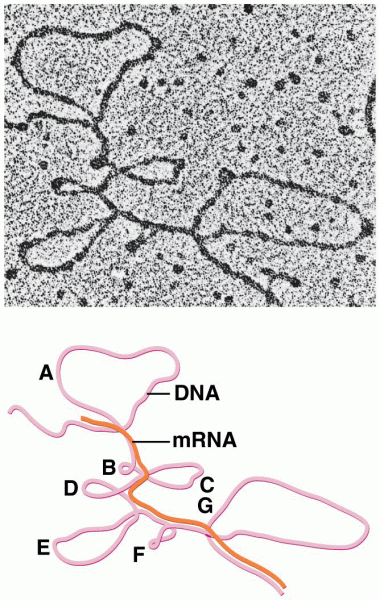
|
|
|
The horizontal fraction bar was introduced by the Arabs.
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.
Walt Disney helped combat malaria by making an animated film in 1943 called The Winged Scourge. This short film starred the seven dwarfs and taught children that mosquitos transmit malaria, which is a very bad disease. It advocated the killing of mosquitos to stop the disease.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.