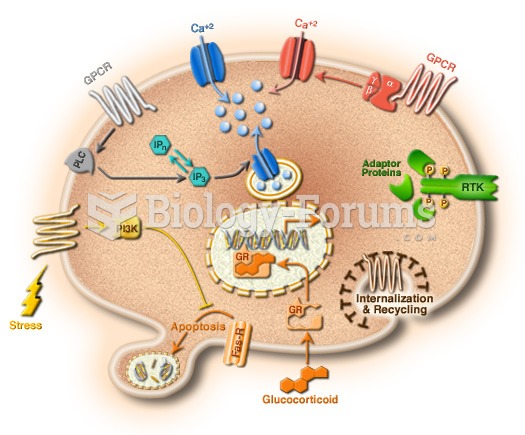
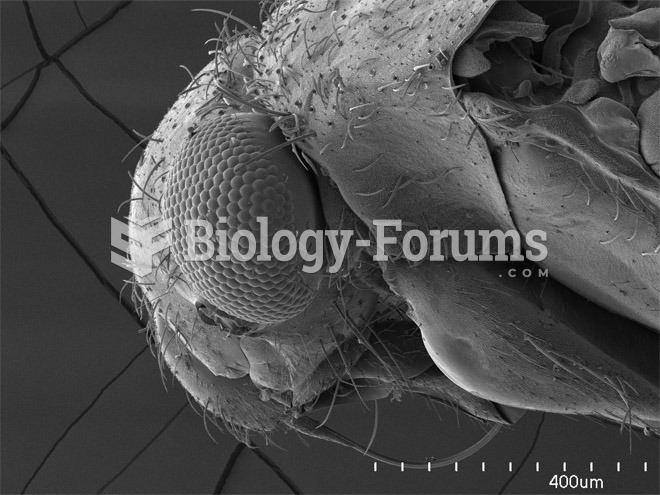
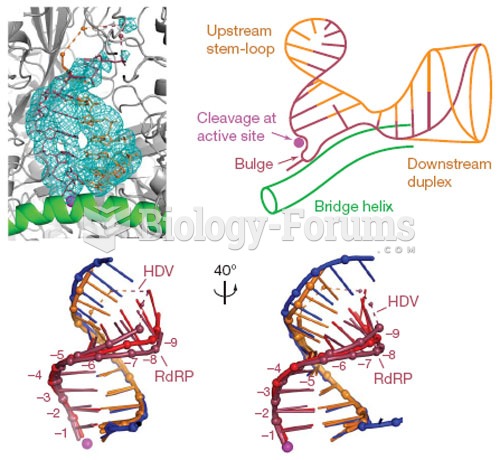
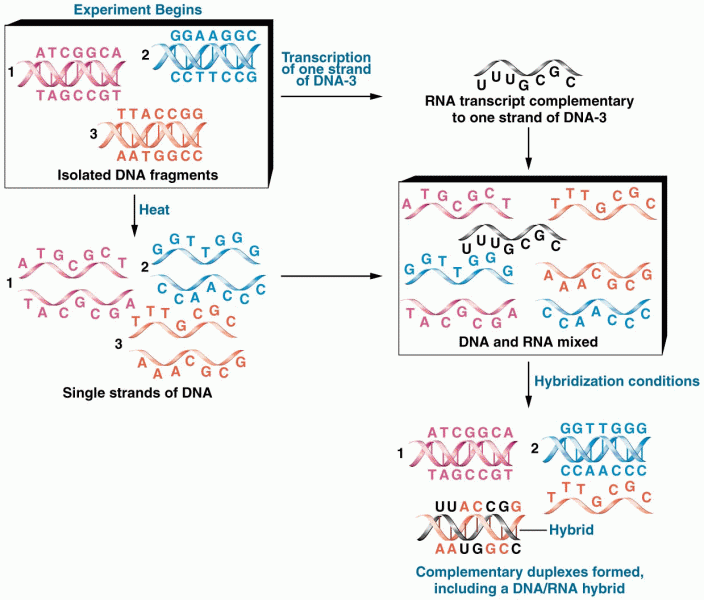
|
|
|
A good example of polar molecules can be understood when trying to make a cake. If water and oil are required, they will not mix together. If you put them into a measuring cup, the oil will rise to the top while the water remains on the bottom.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
Medication errors are three times higher among children and infants than with adults.
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.