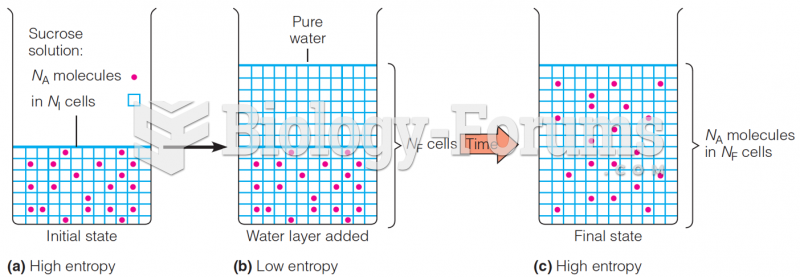
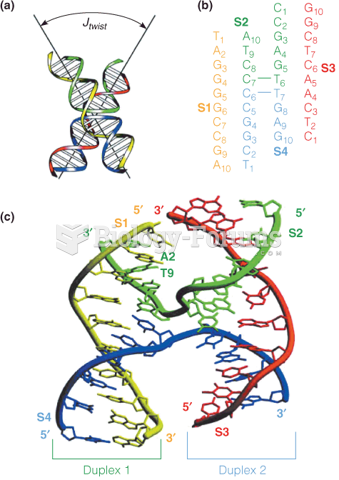
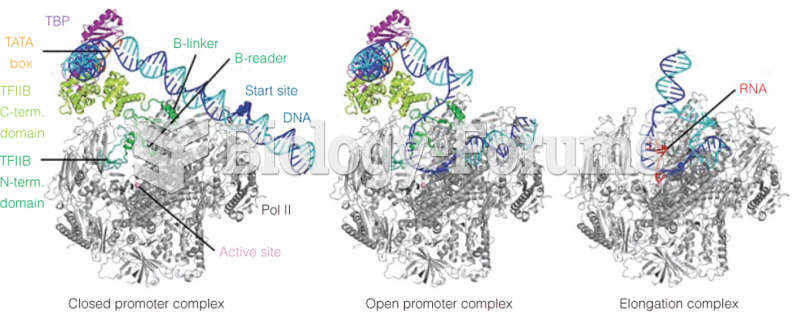
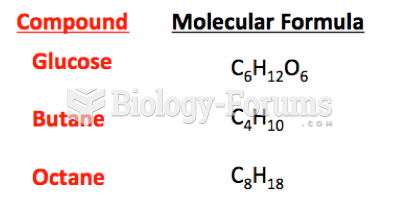
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
Did you know?
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Did you know?
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.