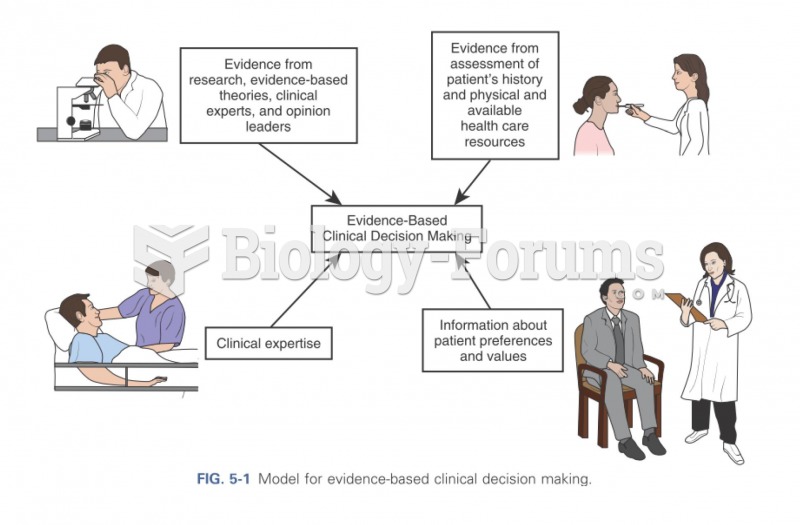
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.
Did you know?
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
Every 10 seconds, a person in the United States goes to the emergency room complaining of head pain. About 1.2 million visits are for acute migraine attacks.
Did you know?
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.