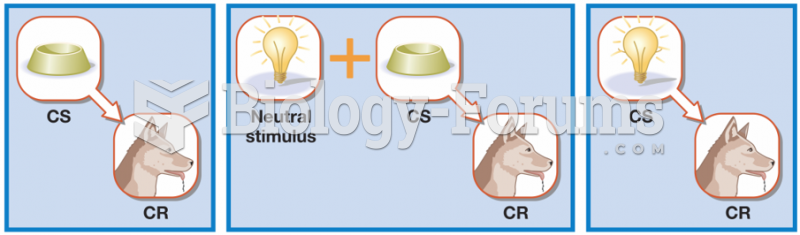
|
|
|
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.
Lower drug doses for elderly patients should be used first, with titrations of the dose as tolerated to prevent unwanted drug-related pharmacodynamic effects.
Human stomach acid is strong enough to dissolve small pieces of metal such as razor blades or staples.
An identified risk factor for osteoporosis is the intake of excessive amounts of vitamin A. Dietary intake of approximately double the recommended daily amount of vitamin A, by women, has been shown to reduce bone mineral density and increase the chances for hip fractures compared with women who consumed the recommended daily amount (or less) of vitamin A.
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
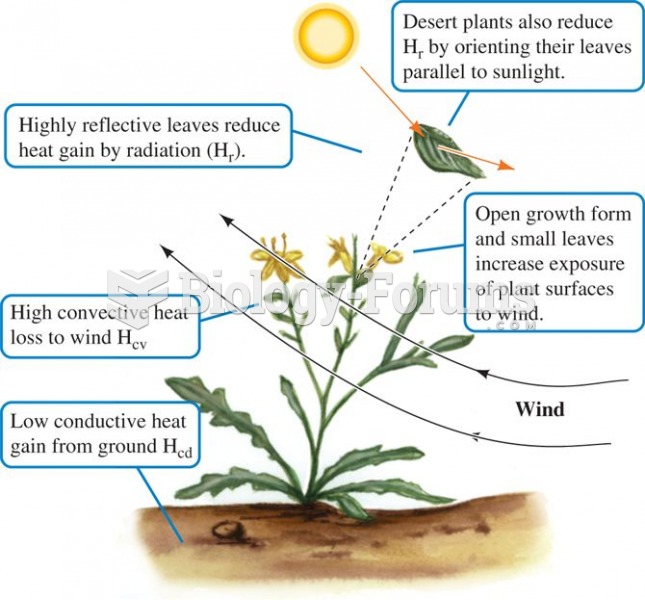 The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
The form and orientation of desert plants reduces heat gain from the environment and facilitates coo
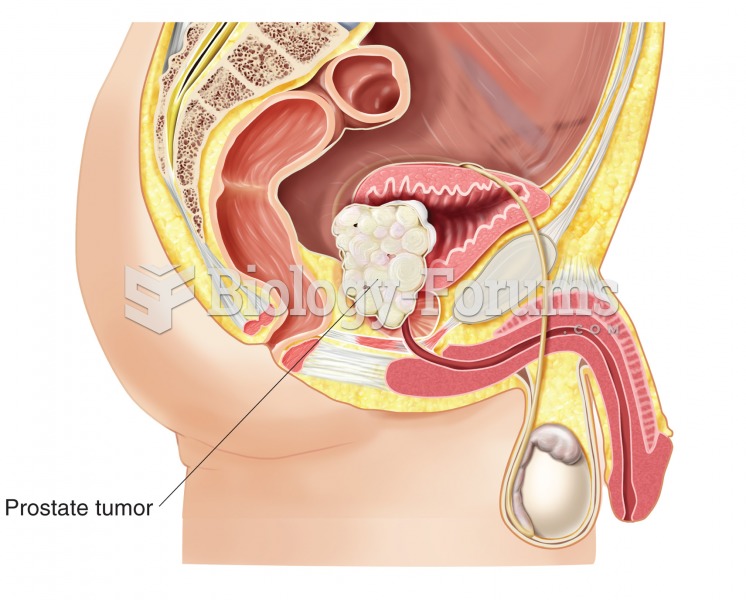 Prostate cancer. In this example, a large mass has grown into the urinary bladder. Prostate cancer i
Prostate cancer. In this example, a large mass has grown into the urinary bladder. Prostate cancer i