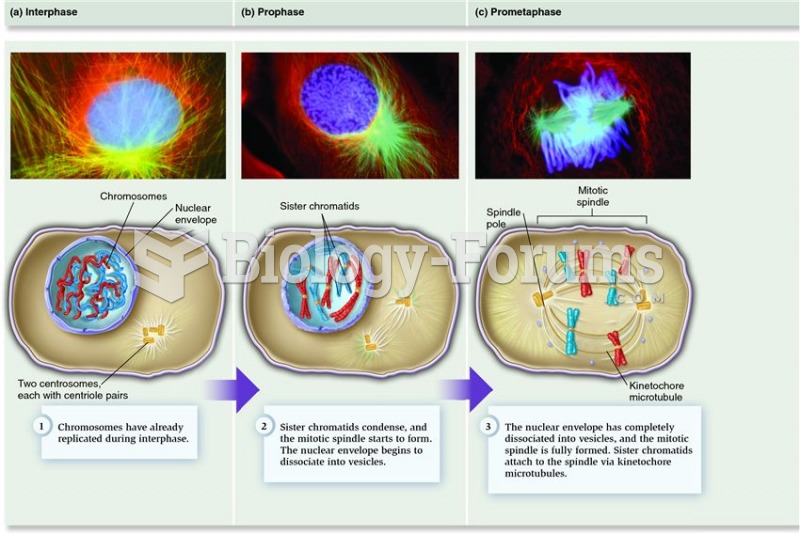
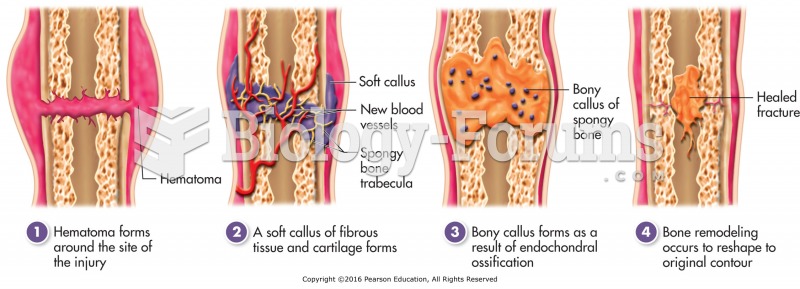
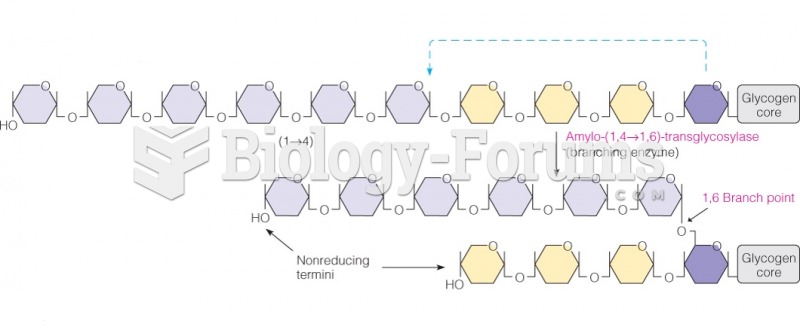
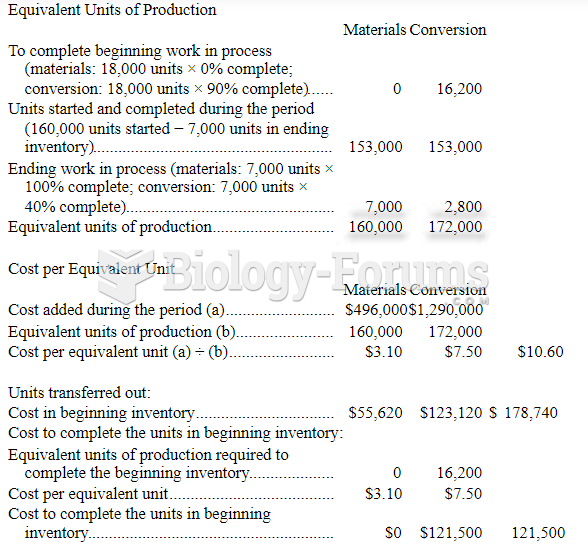
|
|
|
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.