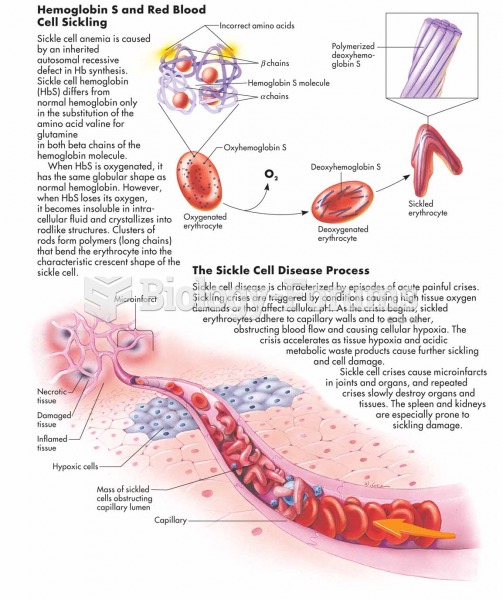
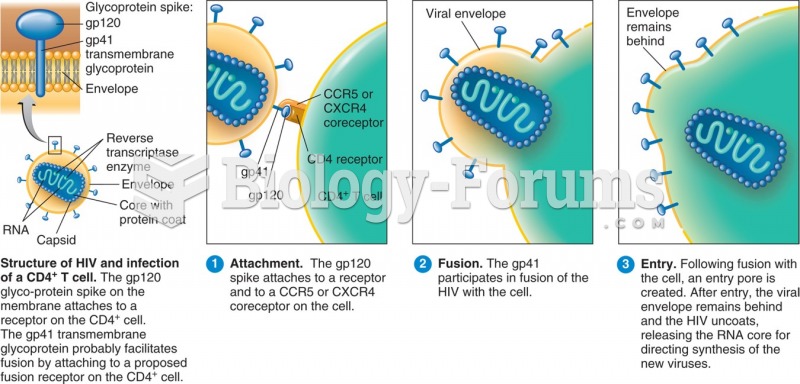
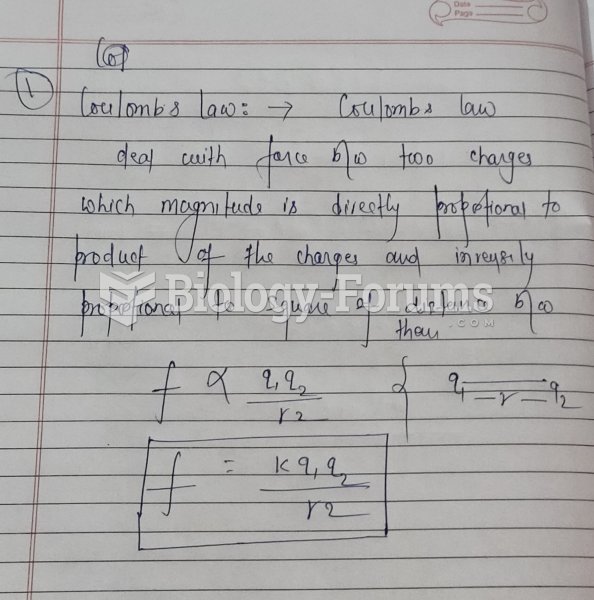
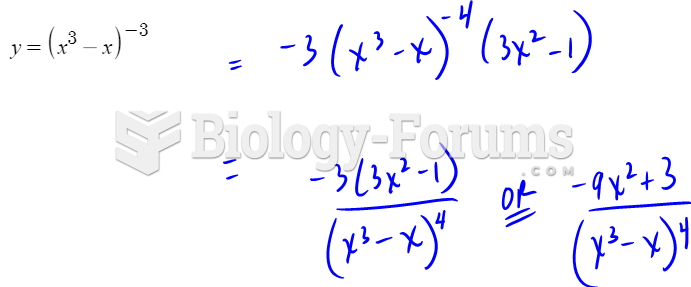|
|
|
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.