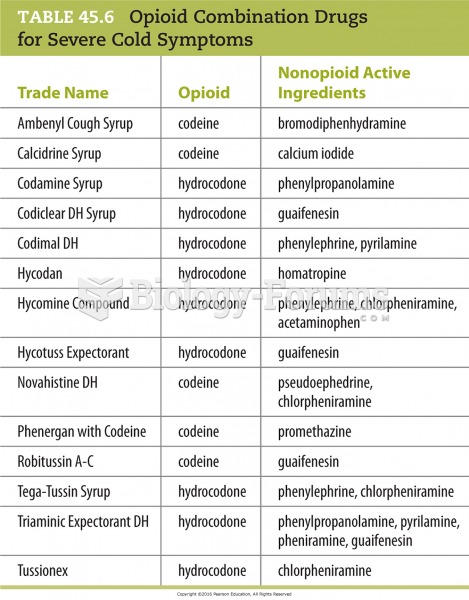
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Adult head lice are gray, about ? inch long, and often have a tiny dot on their backs. A female can lay between 50 and 150 eggs within the several weeks that she is alive. They feed on human blood.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
Hyperthyroidism leads to an increased rate of metabolism and affects about 1% of women but only 0.1% of men. For most people, this increased metabolic rate causes the thyroid gland to become enlarged (known as a goiter).
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.